Carbon Dioxide Electron Arrangement
Also shells dont stack neatly one on top of another so dont always assume an elements valence is determined by the number of electrons in its outer shell. The internal organization of most kinds of leaves has evolved to maximize exposure of the photosynthetic organelles the chloroplasts to light and to increase the absorption of carbon dioxide while at the same time controlling water loss.

Co2 Lewis Structure Easy Hard Science
Carbon is a pattern maker.

. In high concentrations its odor becomes acidic. 3 as organic matter in soils. H 2 is the dominant product with a product purity of 65 over the range of applied potentials 08 to 14 V vs RHE as it is only a 2-electron product whereas C 2 H 4 is a 12-electron product that indicates that it requires six times more energy.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. And 5 in the oceans as. An example of a molecule with this geometry is CH 2 CCH 2 which has two H 2 C-C bonds forming a 180-degree angle.
It can link to itself forming long resilient chains called polymers. Its monatomic form H is the most abundant chemical substance in the Universe constituting. Nitrogen dioxide typically arises via the oxidation of nitric oxide by oxygen in air eg.
It has a molar mass of 44009 gmol 1. It also takes care of the steric number that is the number of regions of electron density surrounding the atom. In the laboratory NO 2 can be prepared in a two-step.
Low electron products like H 2 and CO have an advantage over higher electron products such as CH 4 and C 2 H 4. Nitrogen dioxide is formed in most combustion processes using air as the oxidantAt elevated temperatures nitrogen combines with oxygen to form nitric oxide. It can also bond with up to four other atoms because of its electron arrangement.
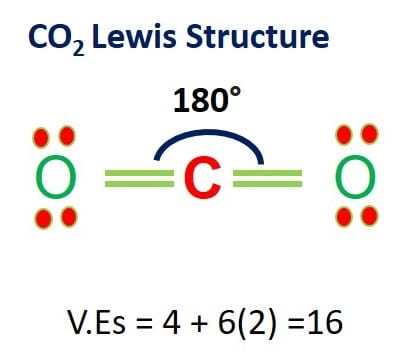
Remember that an elements electron cloud will become more stable by filling emptying or half-filling the shell. Carbon dioxide CO 2 is another linear molecule consisting of two O-C bonds that are 180 degrees apart. Here is a table of element valences.
Useful properties of Carbon dioxide. It is used as a feedstock for the synthesis of chemicals and fuels. CO 2 was found in large concentrations for all test conditions reviewed in the literature as shown in Table 1The major component of first venting gas was CO 2 which was verified in experiments from literature 172628Overheating experiments for over 50 cells also indicated CO 2 as having the highest volume percentage in.
Beryllium Be formerly until 1957 glucinium chemical element the lightest member of the alkaline-earth metals of Group 2 IIa of the periodic table used in metallurgy as a hardening agent and in many outer space and nuclear applications. 4 in the lithosphere as fossil fuels and sedimentary rock deposits such as limestone dolomite and chalk. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table.
2 as the gas carbon dioxide in the atmosphere. 1 2 N 2 O 2 NO 2. On this view the point of the cycle is the accumulation of high-energy electron pairs from small carbon molecules.
Carbon dioxide is used to carbonate sodas. The melting point of CO2 is 566 C. AX 2 E and AX 2 E 2 - If.
Carbon from atmospheric carbon dioxide hydrogen and oxygen from water nitrogen from ammonia and nitrates in the soil and other elements needed in smaller amounts from inorganic salts in the soil. It is soluble in water. Carbon Dioxide CO 2.
They use the energy they derive from sunlight to build these atoms into sugars amino acids. As result of corona discharge. NO 1 2 O 2 NO 2.
Their surfaces are waterproofed by the plant cuticle and gas exchange between the mesophyll cells and the atmosphere is controlled. It is heavier than air. Oxygen O 2 and ATP the form of energy directly employed by cells and tissues to carry.
Plants are able to obtain all the atoms they need from inorganic sources. AX 2 - The two-electron domain structure produces a linear molecule with electron groups 180 degrees apart. Diving Deeper Into the Krebs Cycle Reactions You may notice that two critical molecules expected to be present in aerobic respiration are missing from the Krebs cycle.
Carbon is stored on our planet in the following major sinks 1 as organic molecules in living and dead organisms found in the biosphere. Since each atom has steric number 2 by counting one triple bond and one lone pair the diatomic N2 will be linear in geometry with a bond angle of 180. Mainly the VSEPR model focuses on the electron pairs around the central atoms.
Atomic number 4 atomic weight 90121831 melting point 1287 C 2349 F boiling point 2471 C 4480 F specific gravity 185 at 20 C.

Carbon Dioxide Molecule Co2 Lewis Dot Cross Electronic Diagram Covalent Bonds Ball Stick Space Filling 3d Models Boiling Point Melting Point Doc Brown S Chemistry Revision Notes

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube

Co2 Molecular Geometry And Bond Angles Carbon Dioxide Youtube
Co2 Lewis Structure And Molecular Geometry What S Insight
0 Response to "Carbon Dioxide Electron Arrangement"
Post a Comment